Engineered for flexibility and reliability, the DPT-400S blister line delivers high-performance packaging, allowing you to adapt to changing production needs with ease and confidence.
-
Blisters/Min:
800~1500 blisters/min -
Format area:
220X400mm
Engineered for flexibility and reliability, the DPT-400S blister line delivers high-performance packaging, allowing you to adapt to changing production needs with ease and confidence.
 GET A QUOTE
GET A QUOTE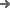
 DOWNLOAD
DOWNLOAD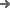
Whether small, medium or large batches, with the LBP-400S blister line, pharmaceutical manufacturers are well positioned for all eventualities. The multi-talent in the DPT family combines high performance with unmatched flexibility, allowing you can respond to unexpected changes in demand in the shortest possible space of time. For maximum process reliability and superior productivity in pharmaceutical packaging.
| Punching frequency | 16-35 time/min 1-12 plate/time |
| Max forming area | 220×400mm |
| Adjustable scope of travel | 50-220mm |
| Specification of blister | Can design asthe requirement of customer |
| Packing material | PVC should conform with GB5663(0.15-0.5)x400mm,the aperture of scrollis 70-76mm, Maximum outer diameter of the roll film: 500-600mm |
| Electric heating power | PVC preheating plate 2×4.5 KW |
| Mould cooling | Tap water or circulating water 60L/h |
| Main motor power | 2KW |
 |
 |
 |
 |
 |
 |



We offer after-sales support to keep your production going.
This includes online advice 24/7, on-site commissioning, GMP guidance, fast response and plenty of spare parts.
Our high-speed vial blister machine achieves a validated output of ≥220 blisters/minute (equivalent to ≥440 vials/minute in double-row configuration) with an OEE ≥88% in continuous three-shift GMP production. This performance is calculated based on: Availability ≥95% (achieved through quick changeover systems and predictive maintenance), Performance ≥97% (maintaining rated speed with minimal speed loss), and Quality ≥99.5% (ensured by integrated vision inspection systems). Our validation protocols include 30-day continuous runs with Cpk ≥1.67 for all critical process parameters, demonstrating consistent performance under production conditions.
We guarantee ≤0.008% breakage rate through our proprietary gentle-handling system that combines: Servo-controlled acceleration profiling limiting mechanical forces to <1.8G, Active vibration damping with real-time frequency analysis, and Precision-guided vial handling using polymer-coated rails. The system is validated using high-speed cameras (1000fps) to analyze vial movement, with data from 20 million vials showing breakage Cpk ≥2.0. Additionally, we implement automated glass fragment detection using 5MP color scan cameras to ensure product safety.
Our dual-technology leak detection system combines high-resolution vacuum decay testing (sensitivity to 3μm leaks per ASTM F2338-09) with laser-based headspace analysis for oxygen ingress detection. The system achieves ≥99.97% detection probability with false-reject rate ≤0.005%, exceeding USP <1207> requirements for sterile products. Each detection cycle includes automatic calibration verification using certified reference standards, with all test data recorded in 21 CFR Part 11 compliant audit trails.
Our RFID-enabled Quick Changeover System achieves complete format change in ≤15 minutes using only standard tools. This includes: blister mold replacement (5 minutes), vial handling system adjustment (4 minutes), and vision system recalibration (6 minutes). Each changeover is validated through three consecutive successful runs with dimensional verification showing ±0.1mm repeatability. The system includes digital work instructions with torque specifications (e.g., 12 N·m ±0.3 N·m for sealing station bolts) to ensure operator-independent repeatability.
Our comprehensive TCO analysis demonstrates 35-40% lower total costs versus conventional solutions, achieved through: Reduced energy consumption (45% lower via regenerative drives), Minimized maintenance costs (30% reduction through predictive maintenance), Lower labor requirements (50% reduction via automation), and Reduced quality losses (60% decrease via advanced inspection). Based on European energy rates (€0.18/kWh), the estimated annual operating cost is €65,200, with typical ROI period of 18-22 months for medium-to-high volume producers.
We implement a multi-level contamination control strategy that includes: Material-level using 316L stainless steel (Ra ≤0.4μm) in all product contact parts; Design-level featuring quick-disconnect components and zero product accumulation zones; Process-level with validated CIP system achieving 4-log reduction in bioburden. Our cleaning validation protocols demonstrate residue levels <0.1 μg/cm² per EMA guidelines, with comprehensive swab testing documentation provided for regulatory submissions.
Our system delivers full 21 CFR Part 11/Annex 11 compliance through: Electronic signatures with dual-factor authentication, Immutable audit trails capturing all parameter changes (including "before-after" values), and Automated data archiving with 10-year retention. The system generates real-time compliance reports showing 100% data integrity across all critical process parameters, following ALCOA+ principles for all electronic records. We provide complete validation packages including IQ/OQ/PQ protocols with pre-approved acceptance criteria.
Our regenerative drive system recovers ≥42% of deceleration energy, reducing total consumption by ≥45% versus conventional systems. Detailed energy monitoring shows: Base load: 18.5 kW, Peak demand: 35.2 kW, with an average consumption of ≤0.085 kWh per 100 blisters. All motors meet IE5 ultra-premium efficiency standards, and we provide ISO 50001 energy performance certificates demonstrating annual savings of €28,500 at European energy rates.
We provide a complete validation package including: User Requirements Specification (URS), Functional Specifications (FS), Design Qualification (DQ), IQ/OQ/PQ protocols with pre-approved acceptance criteria, and Traceability Matrix linking requirements to test cases. Our GAMP 5 Category 4 software validation includes source code review and cybersecurity assessment. We guarantee 48-hour response time for audit support and assign dedicated validation specialists throughout your qualification process.
Our Global Service Network maintains ≥99% spare parts availability from strategically located warehouses in EU, USA, and Asia, with ≤18-hour delivery for critical components. We offer 24/7 multilingual support with ≤10-minute response time for priority issues, backed by ≥97% first-contact resolution rate. All service engineers hold pharmaceutical manufacturing certifications with ≥15 years of experience, and we provide customized SLA agreements with financial penalties for missed response times. Our remote diagnostic system enables 85% of issues to be resolved without on-site visits.

