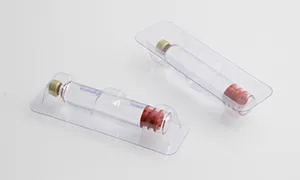
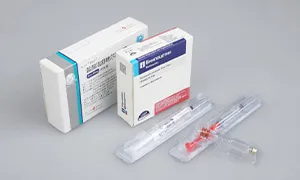
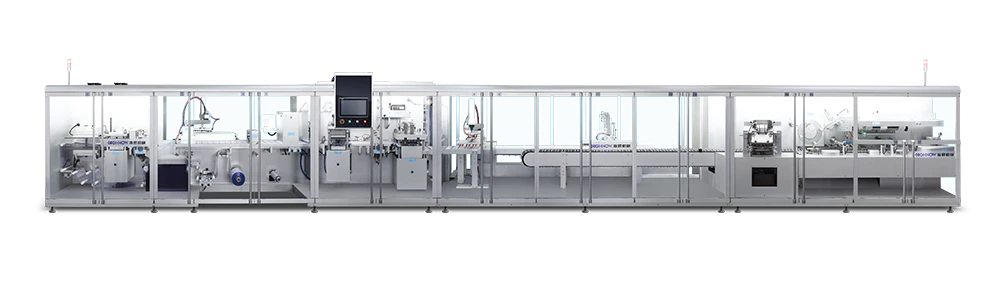
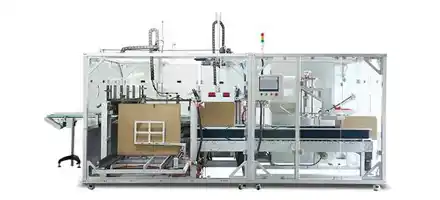
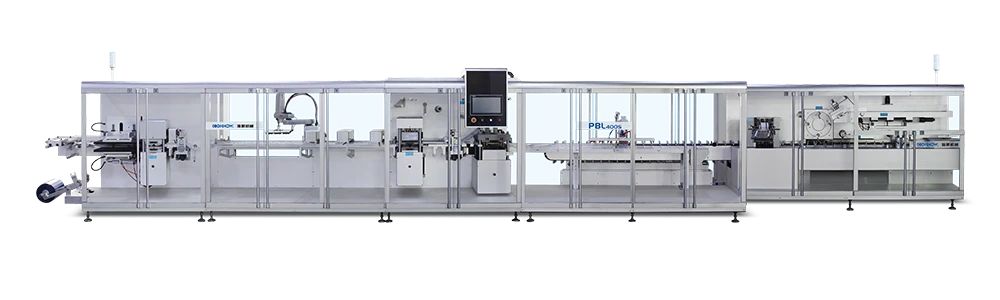
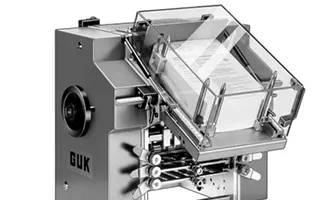
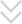The equipment has advanced self-inspection function, automatically recognizing the section of broken blisters, lack of drugs, lack of drug version, lack of instructions, etc. and empty box detection memory, and 100% rejection of waste products.
-
Punching frequency:
16-35 times / minute 1-12 plate/time -
Boxing capacity:
60-80 boxes / min









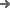




















.webp)