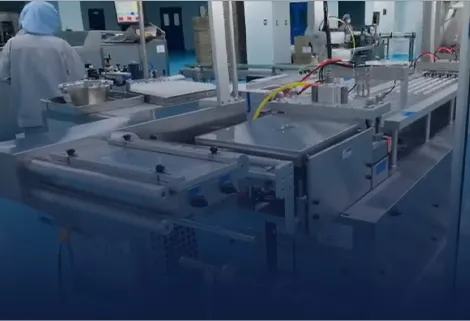
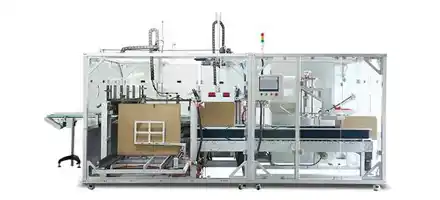
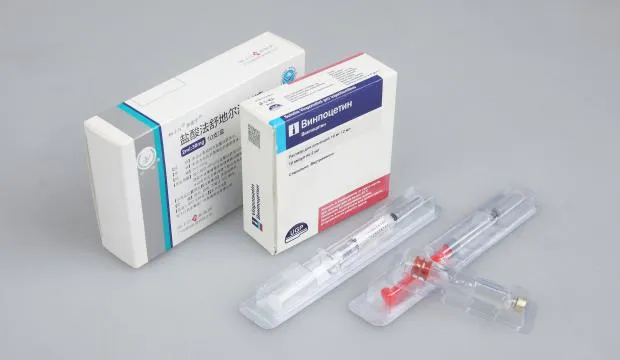
Designed to meet strict sterility requirements, our syringes blister packaging incorporates specialized carriers and vision-assisted alignment. Servo control ensures gentle handling and accurate placement, providing a foolproof solution for pre-filled syringes.









.webp)
.webp)
.webp)
.webp)