What is the maximum validated production speed for ampoule blister packaging while maintaining ≤0.008% breakage rate?
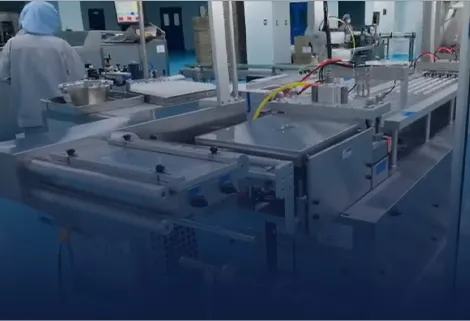
Our ampoule blister packaging line achieves a validated speed of ≥180 blisters per minute (equivalent to 360 ampoules/minute in dual-line configuration) while guaranteeing breakage rate ≤0.008%. This performance is validated through 28-day continuous GMP runs measuring: mechanical stability (vibration levels <2.0 mm/s), thermal consistency (sealing temperature stability ±0.9°C), and handling integrity (force monitoring <1.5G). All critical parameters demonstrate Cpk ≥1.67 with validation data available from 15 million ampoules processed.
How do you prevent micro-crack formation in glass ampoules during high-speed blister packaging?
We prevent micro-cracks through our proprietary gentle-handling system featuring: Servo-controlled acceleration profiling limiting forces to <1.5G, Vibration-isolated conveying with active damping, and Precision guide rails with medical-grade polymer coatings. Validation using acoustic emission testing detects stress levels below glass fracture thresholds, while automated vision inspection identifies micro-cracks ≥25μm with ≥99.95% detection accuracy.
What leak detection methodology ensures ampoule integrity for oxygen-sensitive injectable products?
Our triple-technology inspection system combines: High-sensitivity vacuum decay (detecting leaks ≥2μm per ASTM F2338-09), Laser-based headspace analysis for oxygen ingress (<0.5% detection threshold), and UV fluorescence testing for micro-crack detection. The system achieves ≥99.98% detection probability with false rejection rate ≤0.004%, validated per USP <1207> requirements for sterile injectable products.
How quickly can format changeover be completed between different ampoule sizes (1ml to 20ml)?
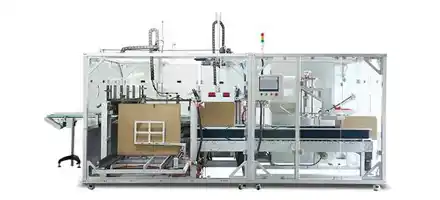
Our RFID-enabled Quick Changeover System achieves complete ampoule size transitions in ≤12 minutes using standard tools. This includes: blister mold replacement (4 minutes), ampoule handling adjustment (3 minutes), vision system recalibration (5 minutes). Validated through 400+ change cycles showing ±0.15mm repeatability, with digital torque specifications (e.g., 10 N·m ±0.2 N·m for sealing station) ensuring consistent performance.
What is the total cost of ownership over 7 years compared to conventional ampoule packaging solutions?
Our comprehensive TCO analysis demonstrates 38-42% lower total costs through: Energy consumption reduction (48% lower via regenerative drives), Maintenance cost optimization (35% reduction via predictive maintenance), Labor efficiency (55% improvement via automation), and Quality loss minimization (65% reduction via advanced inspection). Based on European energy rates (€0.18/kWh), annual operating cost is €58,300 with ROI period of 16-20 months.
How do you ensure particulate contamination control during ampoule blister packaging?
We implement multi-stage contamination control including: ISO Class 5 air shower systems, HEPA-filtered laminar airflow (0.45 m/s ±0.1), and automated particulate monitoring detecting particles ≥5μm. Our validated cleaning protocols achieve surface particle counts <3 particles >0.5μm per cm², meeting USP <788> requirements for injectable products. All product contact surfaces utilize 316L stainless steel (Ra ≤0.3μm) with electropolished finishes.
What level of data integrity and regulatory compliance does your ampoule packaging line provide?
Our system ensures full 21 CFR Part 11/Annex 11 compliance through: Electronic signatures with dual authentication, Immutable audit trails capturing all parameter changes, and Automated data backup with 10-year retention. The ALCOA+ compliant platform demonstrates 100% data integrity across >180 parameters, with real-time compliance reporting and cybersecurity protection per GAMP 5 Category 4 requirements.
How is energy consumption optimized in continuous ampoule packaging operations?
Our energy optimization system achieves consumption of ≤0.078 kWh per 100 blisters through: Regenerative drive technology recovering ≥45% of deceleration energy, IE5 premium efficiency motors reducing base load to 16.2 kW, and Smart thermal management cutting heating energy by 52%. Real-time monitoring shows peak demand of 32.5 kW with ISO 50001 certification validating annual savings of €24,800 at European energy rates.
What validation documentation and support is provided for regulatory submissions?
We deliver complete validation packages including: User Requirements Specification (URS), Functional Specifications (FS), Design Qualification (DQ), IQ/OQ/PQ protocols with predefined acceptance criteria, and Traceability Matrix linking requirements to test cases. Our GAMP 5 compliant software validation includes source code review and cybersecurity assessment. We guarantee 36-hour response for audit support with dedicated validation specialists.
How do you ensure global technical support and spare parts availability for continuous production?
Our Global Support Network maintains ≥99.2% spare parts availability from warehouses in EU, USA, and Asia, with ≤16-hour delivery for critical components. We provide 24/7 multilingual support with ≤8-minute response time for priority issues, backed by ≥98% first-contact resolution rate. All service engineers hold pharmaceutical certifications with ≥12 years experience, supported by SLA agreements with performance guarantees and remote diagnostic capabilities resolving 88% of issues without on-site visits.









2.webp)
.webp)
.webp)
.webp)
2.webp)