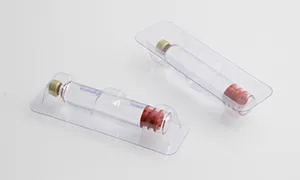
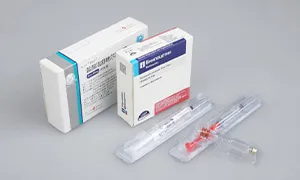
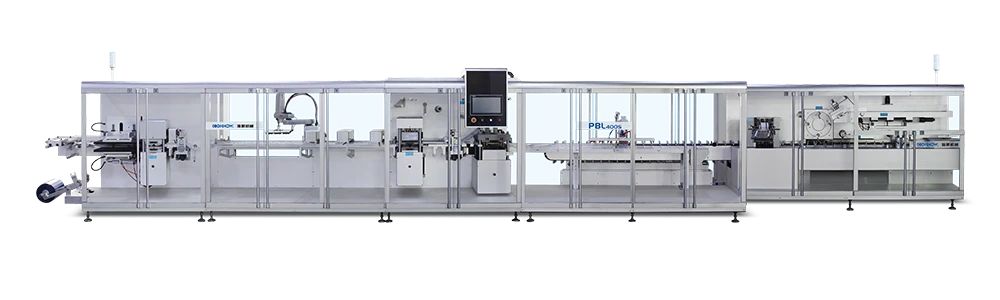
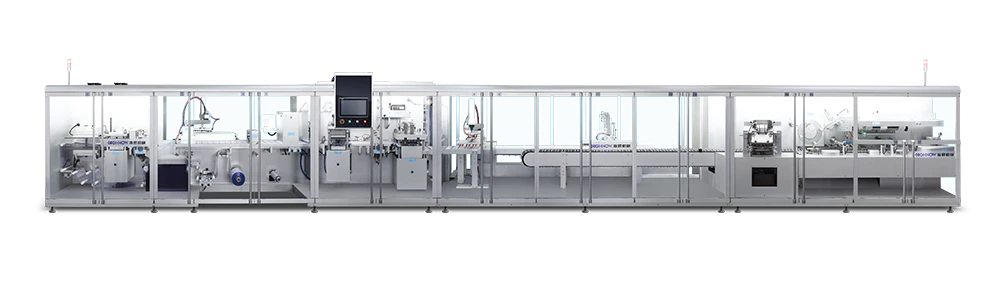
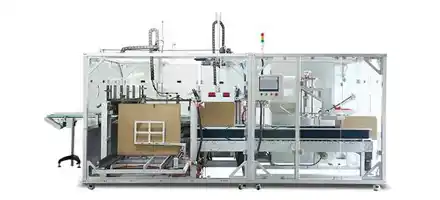
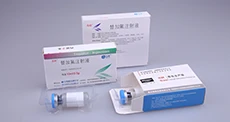
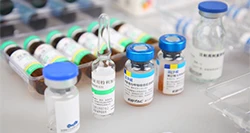
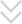According to the latest demand of the market, scientific research technicians of our company research and develop the machine, which is specially for stuffing and box packing of vial. It is applicable for the vertical stuffing of vial and the horizontal boxing packaging is compact and beautiful.
-
Punching frequency:
30 times / minute -
Boxing capacity:
60-80 boxes / min


























.webp)