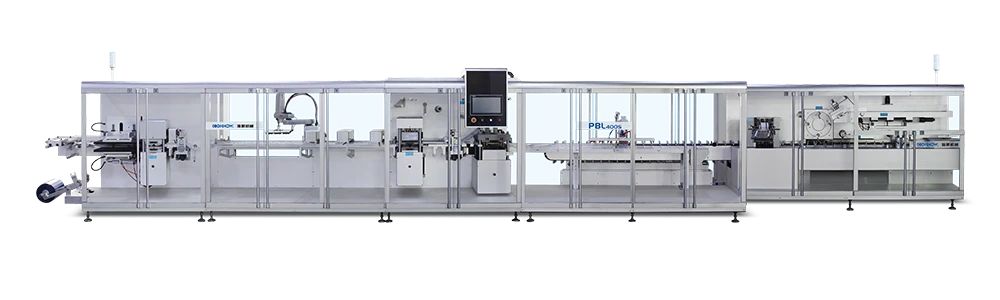
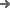The core value of a blister Lines for Vial Product line in two main pillars: "automated integrated control" and "pharmaceutical-grade precision assurance." These two elements simultaneously address three major pain points plaguing pharmaceutical companies: "high-efficiency output," "compliance and traceability," and "flexible adaptability." They are particularly suitable for the mass packaging of various dosage forms of bottled products, such as solid dosage forms and topical ointments.
The question most concerning to buyers—"how to balance compliance and efficiency"—has been clearly answered by authoritative data. According to the "White Paper on Pharmaceutical Packaging Equipment Compliance" published by the China Pharmaceutical Equipment Engineering Association in 2024, blister packaging production lines equipped with servo drive systems have a 41% higher GMP on-site inspection pass rate than equipment using traditional pneumatic systems due to their superior operational stability. This data is not merely theoretical. Previously, a traditional Chinese medicine company adopted this configuration during production line commissioning, reducing the defect rate of its small-volume glass bottle packaging from 0.8% to 0.02%, saving over 2 million RMB in rework costs annually. These tangible benefits earned high recognition from the company's management.
The fundamental difference between ordinary equipment and high-quality solutions lies in scenario adaptability. Taking the production of antibiotic powder injections as an example: bottled products require extremely strict sealing, and any minor oversight can lead to serious consequences. For such situations, we have customized a dual-temperature heat sealing control unit that uses infrared temperature measurement technology to adjust the sealing temperature in real time, ensuring consistent sealing strength across all batches of bottles.
In the health supplement industry, "small-batch, multi-specification" production is common. Long mold changeover times during specification switching can affect production efficiency. Our independently developed quick mold change mechanism reduces the traditional 40-minute mold changeover time to just 12 minutes. Last year, Highnow customized a production line for a health supplement company in Zhejiang, which can simultaneously accommodate three bottle sizes (10ml, 20ml, and 50ml). Specification switching requires no production stoppage, ensuring uninterrupted production.
The key to customized service is not simply adjusting equipment parameters, but conducting an in-depth needs analysis of the entire production process. Our engineers will be on-site for more than three days, comprehensively collecting 18 key data points, including bottle material, maximum capacity, and workshop layout. One impressive case involves a traditional Dai medicine company in Yunnan, where the workshop had unusually high humidity, an operating environment highly prone to equipment malfunctions. We specifically upgraded the equipment's corrosion-resistant stainless steel protection level and installed dehumidifying air curtains, fundamentally solving the problem.
Highnow's service goes far beyond equipment delivery, also providing remote debugging services with a 2-hour response time and quarterly optimization suggestions based on equipment operating data. This "equipment + full-cycle service" model extends the equipment's lifespan from the industry average of 8 years to 12 years, while simultaneously reducing overall operation and maintenance costs by 25%. For pharmaceutical companies, this truly provides a worry-free and efficient solution.