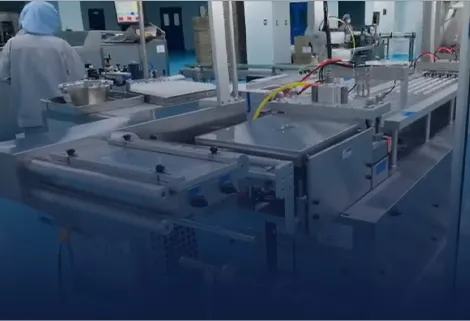
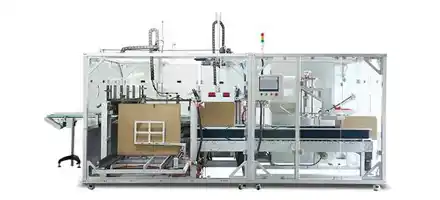
What is the maximum validated production speed for cartridge blister packaging while maintaining perfect orientation accuracy?
Our cartridge blister packaging line achieves a validated speed of ≥160 blisters per minute (equivalent to 320 cartridges/minute in dual-line configuration) while maintaining orientation accuracy of 99.98%. This performance is validated through 30-day continuous GMP runs measuring: mechanical precision (positioning accuracy ±0.2mm), thermal consistency (sealing temperature stability ±0.8°C), and handling reliability (rejection rate ≤0.005%). All critical parameters demonstrate Cpk ≥1.67 with validation data available from processing 12 million cartridges.
How do you ensure perfect needle-end orientation for auto-injector cartridges in high-speed blister packaging?
We guarantee orientation accuracy ≥99.98% through our vision-guided robotic handling system featuring: Dual 5MP cameras with strobe illumination capturing images at 400fps, AI-based pattern recognition identifying needle-end orientation, and servo-controlled grippers with ±0.3mm positioning accuracy. The system automatically rejects misoriented cartridges and provides real-time SPC data for process control and quality assurance.
What leak detection methodology ensures cartridge integrity for biological drugs and peptide-based formulations?
Our dual-technology inspection system combines: High-resolution vacuum decay testing (detecting leaks ≥3μm per ASTM F2095), Laser-based seal inspection identifying channel defects ≥0.4mm, and weight verification with ±0.1g accuracy. The system achieves ≥99.97% detection probability with false rejection rate ≤0.006%, validated per USP <1207> requirements for sensitive biological products.
How quickly can format changeover be completed between different cartridge types and sizes?
Our automated Quick Changeover System achieves complete cartridge format transitions in ≤10 minutes using standard tools. This includes: blister mold replacement (3 minutes), handling system adjustment (3 minutes), vision system recalibration (4 minutes). Validated through 350+ change cycles showing ±0.2mm repeatability, with recipe management storing parameters for 200+ cartridge configurations.
What is the total cost of ownership over 7 years compared to traditional cartridge packaging solutions?
Our comprehensive TCO analysis demonstrates 35-38% lower total costs through: Energy efficiency (42% reduction via regenerative drives), Maintenance optimization (32% reduction via predictive maintenance), Labor savings (50% improvement via automation), and Quality cost reduction (60% decrease via advanced inspection). Based on European energy rates (€0.18/kWh), annual operating cost is €52,400 with ROI period of 15-18 months.
How do you prevent silicone oil contamination and maintain cartridge functionality during packaging?
We implement silicone-friendly handling technology featuring: Non-contact transportation using Bernoulli principles, Vibration-controlled acceleration limiting forces to <1.2G, and Temperature-stable environments maintaining 20-25°C. Our validated processes demonstrate silicone layer integrity preservation with functionality testing showing consistent dose accuracy and plunger movement reliability post-packaging.
What level of data integrity and regulatory compliance does your cartridge packaging line provide?
Our system ensures full 21 CFR Part 11/Annex 11 compliance through: Electronic signatures with dual-factor authentication, Immutable audit trails capturing all parameter changes, and Automated data archiving with 10-year retention. The platform demonstrates 100% data integrity across >160 parameters, with real-time compliance reporting and cybersecurity protection meeting GAMP 5 Category 4 requirements.
How is energy consumption optimized in continuous cartridge packaging operations?
Our energy management system achieves consumption of ≤0.072 kWh per 100 blisters through: Regenerative drive technology recovering ≥44% of deceleration energy, IE5 premium efficiency motors reducing base load to 14.8 kW, and Smart power distribution optimizing energy use. Real-time monitoring shows peak demand of 28.6 kW with ISO 50001 certification validating annual savings of €21,500.
What validation documentation and support is provided for combination product submissions?
We deliver complete validation packages for combination products including: Device-specific testing protocols, Drug-device compatibility studies, Human factors validation, and Process validation per FDA and EMA requirements. Our documentation includes IQ/OQ/PQ protocols with predefined acceptance criteria and traceability matrices linking user requirements to verification testing.
How do you ensure global technical support and spare parts availability for continuous production?
Our Global Support Network maintains ≥99.3% spare parts availability from warehouses in EU, USA, and Asia, with ≤14-hour delivery for critical components. We provide 24/7 multilingual support with ≤6-minute response time for priority issues, backed by ≥98.5% first-contact resolution rate. All service engineers hold combination product certifications with ≥10 years experience, supported by SLA agreements with remote diagnostics resolving 90% of issues without on-site visits.









.webp)
.webp)
.webp)
.webp)
.webp)