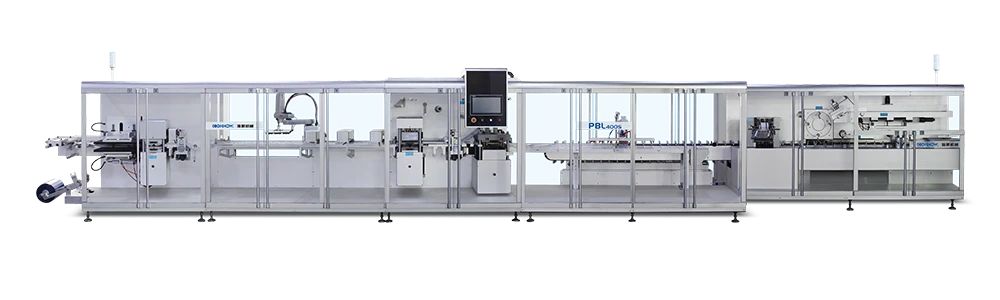
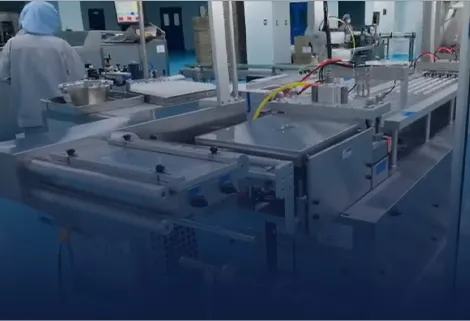
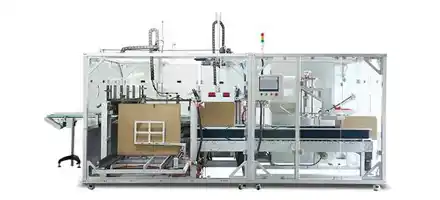
We provide pharmaceutical companies/factories with a full, compliant blister packing-cartoner production system for small bottles, meeting GMP regulations to meet the blister packaging needs of various drugs.
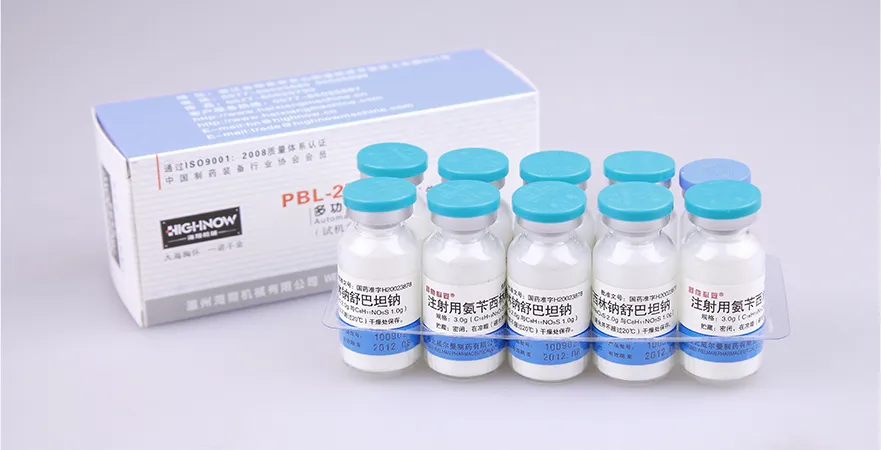
Vial Packing-Cartoner Solutions for Pharmaceuticals