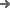Our systems are built with data integrity and traceability as a core feature. The integrated HIGHNOW Plant Monitoring System (PMS) offers Level 3-4 automation, automatically collecting and storing critical process parameters (CPPs) like sealing temperature, pressure, and dwell time. The system features role-based access control, comprehensive audit trails, and electronic signatures that are fully compliant with FDA 21 CFR Part 11 and EU GMP Annex 11. All data is exported in a structured format for easy integration into your QMS, providing complete batch genealogy from raw material to finished blister.