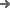We support a seamless and compliant qualification process. Our approach includes:
-
Documentation: We provide a pre-delivery Factory Acceptance Test (FAT) protocol and a comprehensive Documentation Package including User Requirements Specification (URS), Design Qualification (DQ), and Installation Qualification (IQ) templates.
-
On-site Support: Our engineers will support your team during the Installation & Operational Qualification (IQ/OQ) on-site, assisting with the Performance Qualification (PQ) using your actual vials and blisters.
-
Validation: The entire process is designed to meet GAMP 5 guidelines, ensuring a smooth audit by regulatory bodies. We ensure the line performs as specified in your production environment, not just in our factory.