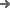Ensuring hermetic seal integrity is non-negotiable for parenteral products. The HIGHNOW line integrates a multi-stage, in-process control system. It begins with a precision thermal control system for the lidding foil, maintaining temperature within ±1.5°C to prevent micro-fissures. This is followed by a double-sided vacuum leak testing station that subjects each blister cavity to a test sensitivity of up to 5x10⁻⁶ mbar·L/s. We can provide the validation protocols and SOP templates for this test, aligning with USP <1207> guidelines. For critical applications, we offer an optional High Voltage Leak Detection (HVLD) module to non-destructively detect seal flaws as small as 2 microns.